1. Powolny A, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–314. [PMC free article] [PubMed]
2. Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–181. [PubMed]
3. Fleischauer A, Arab L. Garlic and Cancer: A critical review of the epidemiologic literature. J. Nutr. 2001;131:1032S–1040S. [PubMed]
4. Herman-Antosiewicz A, Singh SV. Signal transduction pathways leading to cell cycle arrest and apoptosis induction in cancer cells by Allium vegetable derived organosulfur componds: a review. Mut. Res. 2004;555:121–131. [PubMed]
5. Block E, Ahmad S, Catalfamo JL, Jain M K, Apitz-Castro R. The chemistry of alkyl thiosulfinate esters.9. Antithrombotic organosulfur compounds from garlic: structural mechanistic and synthetic studies. J. Am. Chem. Soc. 1986;108 (22): 7045–7055.
6. Block E, Naganathan S, Putman D, Zhao S-H. Allium Chemistry: HPLC analysis of thiosulfinates from onion, garlic, wild garlic (ramsoms): leek scallion shallot elephant (great headed) garlic, chive, and Chinese chive.Uniquely high allyl to methyl ratios in some garlic samples. J. Agric. Food. Chem. 1992;40:2418–2430.
7. Lee SN, Kim NS, Lee DS. Comparative study of extraction techniques for determination of garlic flavor components by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2003;377(4): 749–756. [PubMed]
8. Laakso I, Seppanen-Laakso T, Hiltunen R, Muller B, Jansen H, Knobloch K. Volatile garlic odor components: gas phases and adsorbed exhaled air analysed by headspace gas chromatography-mass spectrometry. Planta Med. 1989;55 (3): 257–261. [PubMed]
9. Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis P A, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason Sonochem. 2006;13 (1): 54–60. [PubMed]
10. Naznin M, Akagawa M, Okukawa K, Maeda T, Morita N. Characterization of E- and Z-ajoene obtained from different varieties of garlics. Food Chem. 2008;106:1113–1119.
11. Egen-Schwind C, Eckard R, Kemper F H. Metabolism of garlic constituents in the isolated perfused rat liver. Planta Med. 1991;58:301–305. [PubMed]
12. Lawson LD, Ransom DK, Hughes BG. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb. Res. 1992;65 (2): 141–156. [PubMed]
13. Minami T, Boku T, Inada K, Morita I, Okazaki Y. Odor components of human breath after the ingestion of grated raw garlic. J. Food Sci. 1989;54 (3): 763–764.
14. Rosen RT, Hiserodt RD, Fukuda EK, Riuiz RJ, Zhou Z, Lech J, Rosen SL, Hartman TG. Determination of allicin s-allylcysteine and volatile metabolites of garlic in breath, plasma or simulated gastric fluids. J. Nutr. 2001;131:968S–971S. [PubMed]
15. Taucher J, Hansel A, Jordan A, Lindinger W. Analysis of compounds in human breath after ingestion of garlic using proton-transfer-reaction mass spectrometry. J. Agric. Food Chem. 1996;44:3778–3782.
16. Naganawa R, Iwata N, Ishikawa K, Fukuda H, Fujino T, Suzuki A. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl. Environ. Microbiol. 1996;62:4238–4242. [PMC free article] [PubMed]
17. Kaschula CH, Hunter R, Stellenboom N, Caira MR, Winks S, Oqunleye T, Richards P, Cotton J, Zilbeyaz K, Wang Y, Siyo V, Ngarande E, Parker MI. Structure-activity studies on the anti-proliferation activity of ajoene analogues in WHCO1 oesophageal cancer cells. Eur. J. Med. Chem. 2012;50:236–254. [PubMed]
18. Gallwitz H, Bonse S, Martinez-Cruz A, Schlichting I, Schumacher K, Krauth-Siegel RL. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and trypanosoma cruzi trypanothione reductase: crystallographic kinetic and spectroscopic studies. J. Med. Chem. 1999;42 (3): 364–372. [PubMed]
19. Kaschula CH, Hunter R, Parker MI, Hassan HT, Stellenboom N, Cotton J, Zhai XQ. Synthesis and anti-proliferation efficacy of synthetic ajoene analogues on cancer cell-lines. Anti-Cancer Agents Med. Chem. 2011;11 (3): 260–266. [PubMed]
20. Hosono T, Fukao T, Ogihara J, Ito Y, Shiba H, Seki T, Ariga T. Diallyl trisulfidesuppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of b-tubulin J. Biol. Chem. 2005;280 (50): 4147–4193. [PubMed]
21. Li M, Ciu J-R, Ye Y, Min J-M, Zhang L-H, Wang K, Gares M, Cros J, Wright M, Leung-Track J. Antitumor activity of Z-ajoene a natural compound purified from garlic: antimitotic and microtubule-interaction properties. Carcinogenesis. 2002;23 (4): 573–579. [PubMed]
22. Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30 (11): 1191–1212. [PubMed]
23. Kaschula CH, Hunter R, Parker MI. Garlic-derived anti-cancer agents: structure and biological activity of ajoene. Biofactors. 2010;36:78–85. [PubMed]
24. You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, Henderson BE, Fraumeni JF, Jr., Wang TG. Allium vegetables and reduced risk of stomach cancer. J. Natl. Cancer Inst. 1989;81 (2): 162–164. [PubMed]
25. Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Biserni R, Cipriani F, Cocco P, Giacosa A, Marubini E, Puntoni R, Vindigni C, Fraumeni J, Blot W. A case-control study of gastric cancer and diet in Italy. Int. J. Cncer. 1989;44 (4): 611–616. [PubMed]
26. Kim JY, Kwon O. Garlic intake and cancer risk: An analysis using the food and drug administration’s evidence-based review system for the scientific evaluation of health claims. Am. J. Clin. Nutr. 2009;89 (1): 257–264. [PubMed]
27. Ngo SN, Williams DB, Cobiac L, Head RJ. Does garlic reduce risk of colorectal cancer? A systematic review. J. Nutr. 2007;137 (10): 2264–2269. [PubMed]
28. Wargovich MJ, Woods C, Eng VW, Stephens LC, Gray K. Chemoprevention of N-nitrosomethylbenzylamine-induced esophageal cancer in rats by the naturally occurring thioether diallyl sulfide. Cancer Res. 1988;48 (23): 6872–6875. [PubMed]
29. Wattenberg LW, Sparnins VL, Barany G. Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res. 1989;49 (10): 2689–2692. [PubMed]
30. Sparnins VL, Venegas PL, Wattenberg LW. Glutathione S-transferase activity: enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents. J. Natl. Cancer Inst. 1982;68 (3): 493–496. [PubMed]
31. Reddy BS, Rao CV, Rivenson A, Kelloff G. Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Res. 1993;53:3493–3498. [PubMed]
32. Singh A, Shukla Y. Antitumor activity of diallyl sulfide on polycyclic aromatic hydrocarbon-induced mouse skin carcinogenesis. Cancer Lett. 1998;131 (2): 209–214. [PubMed]
33. Mehta RG, Steele V, Kelloff GJ, Moon RC. Influence of thiols and inhibitors of prostaglandin biosynthesis on the carcinogen-induced development of mammary lesions in vitro. Anticancer Res. 1991;11:587–592. [PubMed]
34. Cohen LA, Zhao Z, Pittman B, Lubet R. S-allylcysteine a garlic constituent fails to inhibit N-methylnitrosourea-induced rat mammary tumorigenesis. Nutr. Cancer. 1999;35 (1): 58–63. [PubMed]
35. Schaffer EM, Liu JZ, Green J, Dangler CA, Milner JA. Garlic and associated allyl sulfur components inhibit N-methyl-N-nitrosourea induced rat mammary carcinogenesis. Cancer Lett. 1996;102 (1-2): 199–204. [PubMed]
36. Brady JF, Ishizaki H, Fukuto JM, Lin MC, Fadel A, Gapac JM, Yang CS. Inhibition of cytochrome P-450 2E1 by diallyl sulfide and its metabolites. Chem. Res. Toxicol. 1991;4 (6): 642–647. [PubMed]
37. Park K A, Kweon S, Choi H. Anticarcinogenic effect and modification of cytochrome P450 2E1 by dietary garlic powder in diethylnitrosamine-initiated rat hepatocarcinogenesis. J. Biochem. Mol. Biol. 2002;35 (6): 615–622. [PubMed]
38. Hatono S, Jimenez A, Wargovich MJ. Chemopreventive effect of S-allylcysteine and its relationship to the detoxification enzyme glutathione S-transferase. Carcinogenesis. 1996;17 (5): 1041–1044. [PubMed]
39. Singh SV, Pan SS, Srivastava SK, Xia H, Hu X, Zaren HA, Orchard JL. Differential induction of NAD(P)H:quinone oxidoreductase by anti-carcinogenic organosulfides from garlic. Biochem. Biophys. Res. Commun. 1998;244 (3): 917–920. [PubMed]
40. Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM. Antimutagenic activity of organosulfur compounds from Allium is associated with phase II enzyme induction. Mutat. Res. 2001;495 (1-2): 135–145. [PubMed]
41. Rose P, Whiteman M, Moore P K, Zhun Zhu Y. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potenial therapeutic agents. Nat. Prod. Rep. 2005;22:351–368. [PubMed]
42. Sundaresan S, Subramanian P. Prevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by S-allylcysteine. Mol. Cell Biochem. 2008;310 (1-2): 209–214. [PubMed]
43. Gudi VA, Singh SV. Effect of diallyl sulfide, a naturally occurring anti-carcinogen, on glutathione-dependent detoxification enzymes of female CD-1 mouse tissues. Biochem. Pharmacol. 1991;42 (6): 1261–1265. [PubMed]
44. Perchellet JP, Perchellet EM, Abney NL, Zirnstein JA, Belman S. Effects of garlic and onion oils on glutathione peroxidase activity the ratio of reduced/oxidized glutathione and ornithine decarboxylase induction in isolated mouse epidermal cells treated with tumor promoters. Cancer Biochem. Biophys. 1986;8 (4): 299–312. [PubMed]
45. Ameen M, Musthapa MS, Abidi P, Ahmad I, Rahman Q. Garlic attenuates chrysotile-mediated pulmonary toxicity in rats by altering the phase I and phase II drug metabolizing enzyme system. J. Biochem. Mol. Toxicol. 2003;17 (6): 366–371. [PubMed]
46. Pinto J T, Qiao C, Xing J, Rivlin RS, Protomastro ML, Weissler ML, Tao Y, Thaler H, Heston WD. Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture. Am. J. Clin. Nutr. 1997;66 (2): 398–405. [PubMed]
47. Dirsch VM, Gerbes AL, Volmar AM. Ajoene a compound of garlic induces apoptosis in human promyleoleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kB. Mol. Pharmacol. 1998;53:402–407. [PubMed]
48. Kwon KB, Yoo SJ, Ryu DG, Yang JY, Rho HW, Kim JS, Park JW, Kim HR, Park BH. Induction of apoptosis by diallyl disulfide through activation of caspase-3 in human leukemia HL-60 cells. Biochem. Pharmacol. 2002;63 (1): 41–47. [PubMed]
49. Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003;63 (18): 5940–5949. [PubMed]
50. Kelkel M, Cerella C, Mack F, Schneider T, Jacob C, Schumacher M, Dicato M, Diederich M. ROS-independent JNK activation and multisite phosphorylation of Bcl-2 link diallyl tetrasulfide-induced mitotic arrest to apoptosis. Carcinogenesis. 2012;33 (11): 2162–2171. [PubMed]
51. Yang J-Y, Della-Fera M, Hausman DB, Baile CA. Enhancement of ajoene-induced apoptosis by conjugated linoleic acid in 3T3-L1 adipocytes. Apoptosis. 2007;12:1117–1128. [PubMed]
52. Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin. Cancer Biol. 2006;16 (1): 38–52. [PubMed]
53. Kanterman J, Sade-Feldman M, Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin. Cancer Biol. 2012;22 (4): 307–318. [PubMed]
54. Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007;117 (5): 1175–1183. [PMC free article] [PubMed]
55. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144 (5): 646–674. [PubMed]
56. Jakobisiak M, Lasek W, Golab J. Natural mechanisms protecting against cancer. Immunol. Lett. 2003;90 (2-3): 103–122. [PubMed]
57. Mocellin S, Bronte V, Nitti D. Nitric oxide a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 2007;27 (3): 317–352. [PubMed]
58. Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011;89 (6): 873–891. [PMC free article] [PubMed]
59. Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14 (11): 879–897. [PubMed]
60. Kleinert H, Schwarz PM, Forstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 2003;384 (10-11): 1343–1364. [PubMed]
61. Lamm DL, Riggs DR. Enhanced immunocompetence by garlic: role in bladder cancer and other malignancies. J. Nutr. 2001;131 (3s ): 1067S–1070S. [PubMed]
62. Kyo E, Uda N, Suzuki A, Kakimoto M, Ushijima M, Kasuga S, Itakura Y. Immunomodulation and antitumor activities of aged garlic extract. Phytomedicine. 1998;5 (4): 9. [PubMed]
63. Kyo E, Uda N, Kasuga S, Itakura Y. Immunomodulatory effects of aged garlic extract. J. Nutr. 2001;131 (3s ): 1075S–1079S. [PubMed]
64. Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, Fukuda S, Morimoto K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006;136 (3 Suppl ): 816S–820S. [PubMed]
65. Kuttan G. Immunomodulatory effect of some naturally occuring sulphur-containing compounds. J. Ethnopharmacol. 2000;72 (1-2): 93–99. [PubMed]
66. Bhattacharyya M, Girish GV, Karmohapatra SK, Samad SA, Sinha AK. Systemic production of IFN-alpha by garlic (Allium sativum) in humans. J. Interferon Cytokine Res. 2007;27 (5): 377–382. [PubMed]
67. Hodge G, Hodge S, Han P. Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry . 2002;48 (4): 209–215. [PubMed]
68. Makris A, Thornton CE, Xu B, Hennessy A. Garlic increases IL-10 and inhibits TNFalpha and IL-6 production in endotoxin-stimulated human placental explants. Placenta. 2005;26 (10): 828–834. [PubMed]
69. Keiss HP, Dirsch VM, Hartung T, Haffner T, Trueman L, Auger J, Kahane R, Vollmar AM. Garlic (Allium sativum L. modulates cytokine expression in lipopolysaccharide-activated human blood thereby inhibiting NF-kappaB activity. J. Nutr. 2003;133 (7): 2171–2175. [PubMed]
70. Chang HP, Chen YH. Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition. 2005;21 (4): 530–536. [PubMed]
71. Ide N, Lau BH. Garlic compounds minimize intracellular oxidative stress and inhibit nuclear factor-kappa b activation. J. Nutr. 2001;131 (3s ): 1020S–1026S. [PubMed]
72. Bruck R, Aeed H, Brazovsky E, Noor T, Hershkoviz R. Allicin the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int. 2005;25 (3): 613–621. [PubMed]
73. Salman H, Bergman M, Bessler H, Punsky I, Djaldetti M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. Int. J. Immunopharmacol. 1999;21 (9): 589–597. [PubMed]
74. Liu KL, Chen HW, Wang RY, Lei YP, Sheen LY, Lii CK. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-kappaB activation in RAW 264. macrophages. J. Agric. Food Chem. 2006;54 (9): 3472–3478. [PubMed]
75. Dirsch VM, Kiemer AK, Wagner H, Vollmar AM. Effect of allicin and ajoene, two compounds of garlic, on inducible nitric oxide synthase. Atherosclerosis. 1998;139 (2): 333–339. [PubMed]
76. Chang HP, Huang SY, Chen YH. Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. J. Agric. Food Chem. 2005;53 (7): 2530–2534. [PubMed]
77. Dirsch VM, Vollmar AM. Ajoene a natural product with non-steroidal anti-inflammatory drug (NSAID)-like properties?. Biochem. Pharmacol. 2001;61 (5): 587–593. [PubMed]
78. Ho SE, Ide N, Lau BH. S-allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine. 2001;8 (1): 39–46. [PubMed]
79. Amagase H, Petesch B L, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J. Nutr. 2001;131 (3s ): 955S–962S. [PubMed]
80. Ferguson LR, Philpott M. Cancer prevention by dietary bioactive components that target the immune response. Curr. Cancer Drug Targets. 2007;7 (5): 459–464. [PubMed]
81. Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J. Natl. Cancer Inst. 2007;99 (8): 608–615. [PubMed
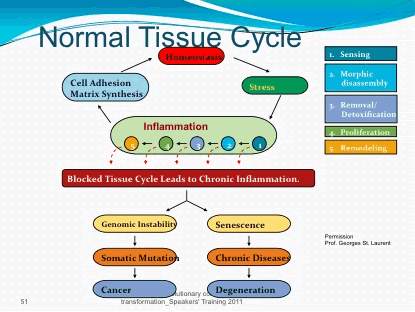
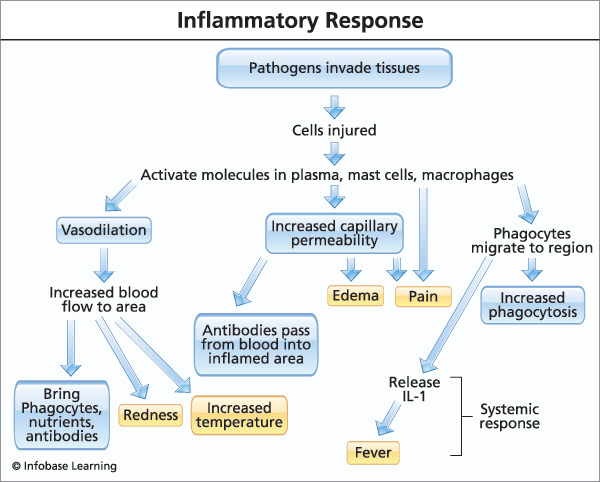
 up and then dies down, there’s nothing to worry about But chronic inflammation can actually be a symptom that causes problems of its own
up and then dies down, there’s nothing to worry about But chronic inflammation can actually be a symptom that causes problems of its own  Soybean, peanut, and “vegetable” oil are all sources of Omega-6 polyunsaturated fat (PUFA) to avoid.
Soybean, peanut, and “vegetable” oil are all sources of Omega-6 polyunsaturated fat (PUFA) to avoid. Garlic
Garlic Vitamin Agent The Health & Naturalistic Source
Vitamin Agent The Health & Naturalistic Source